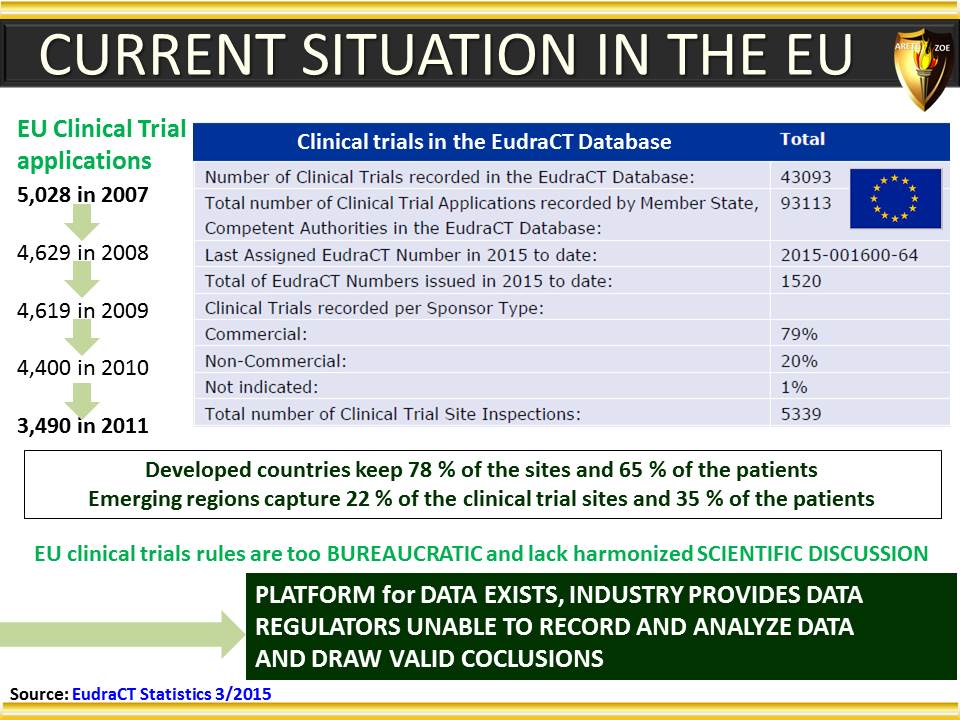
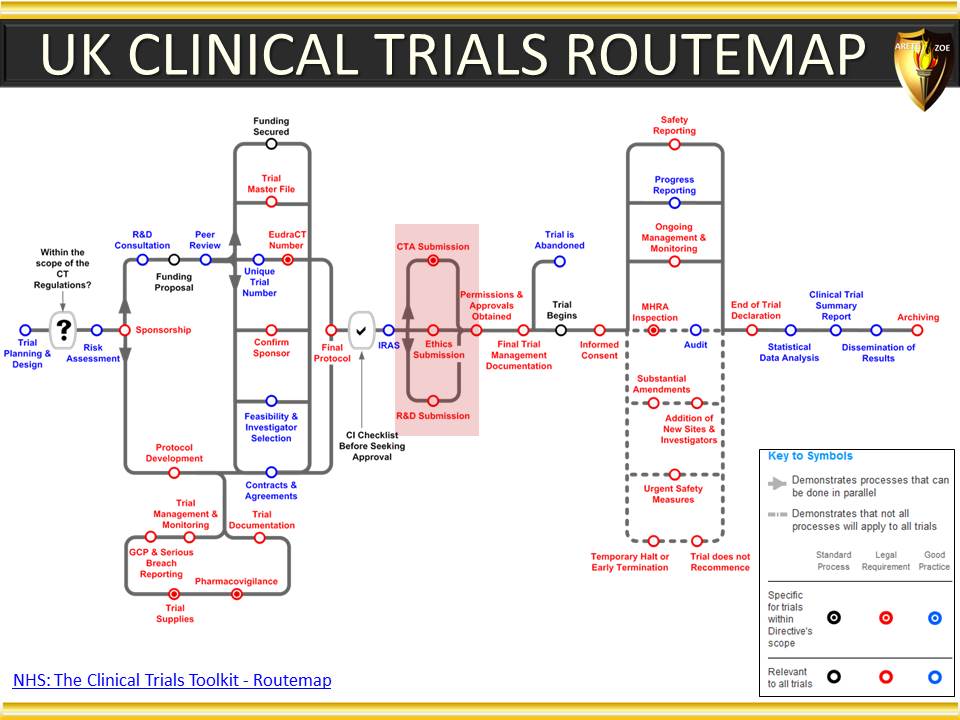
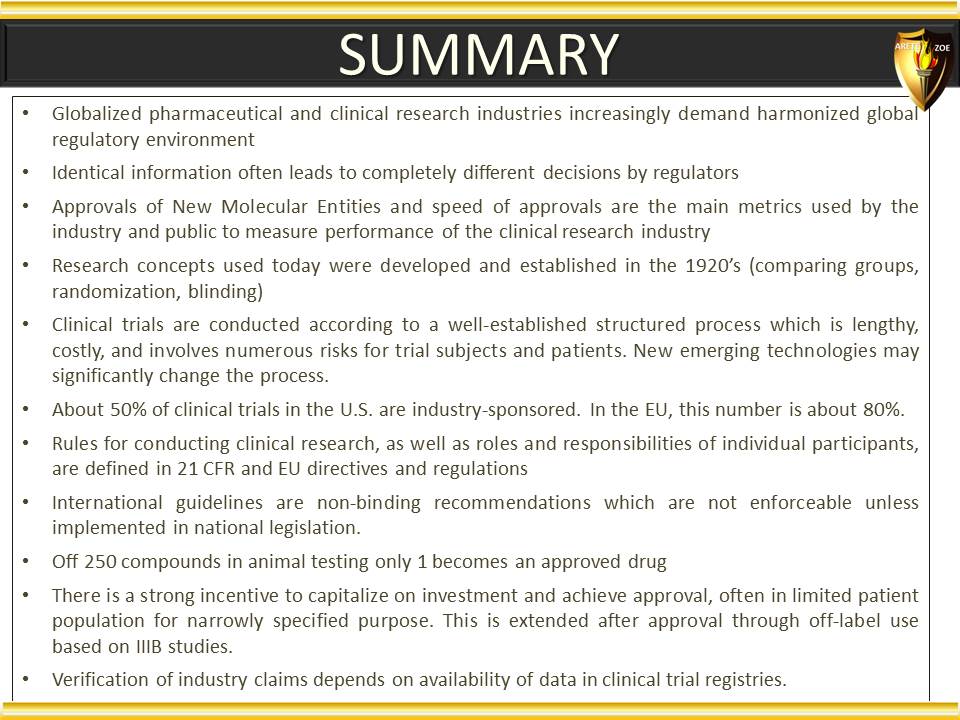
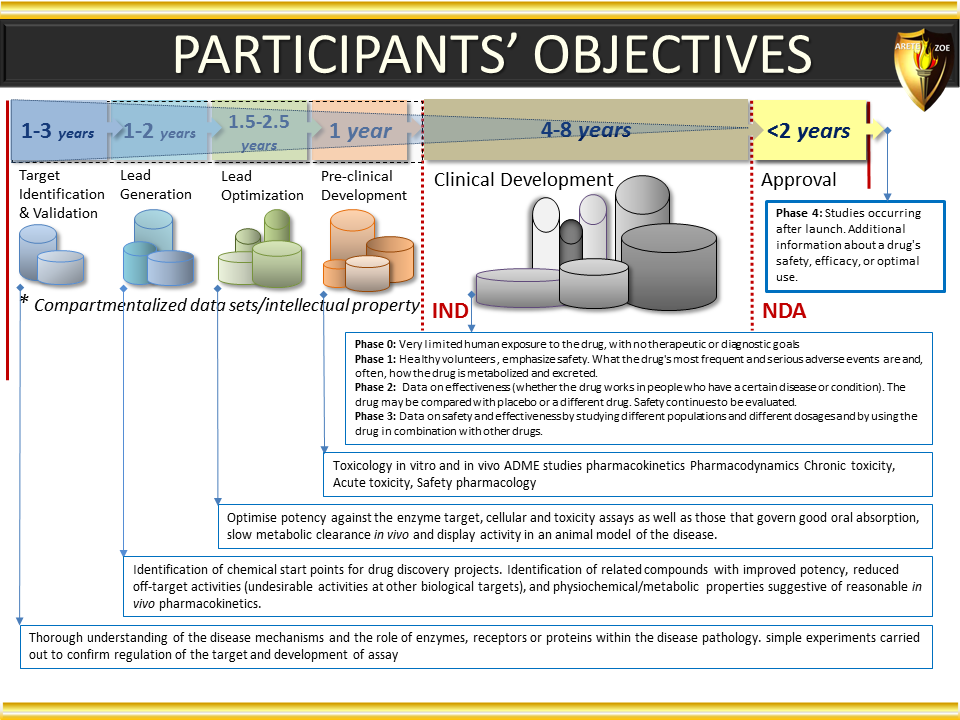
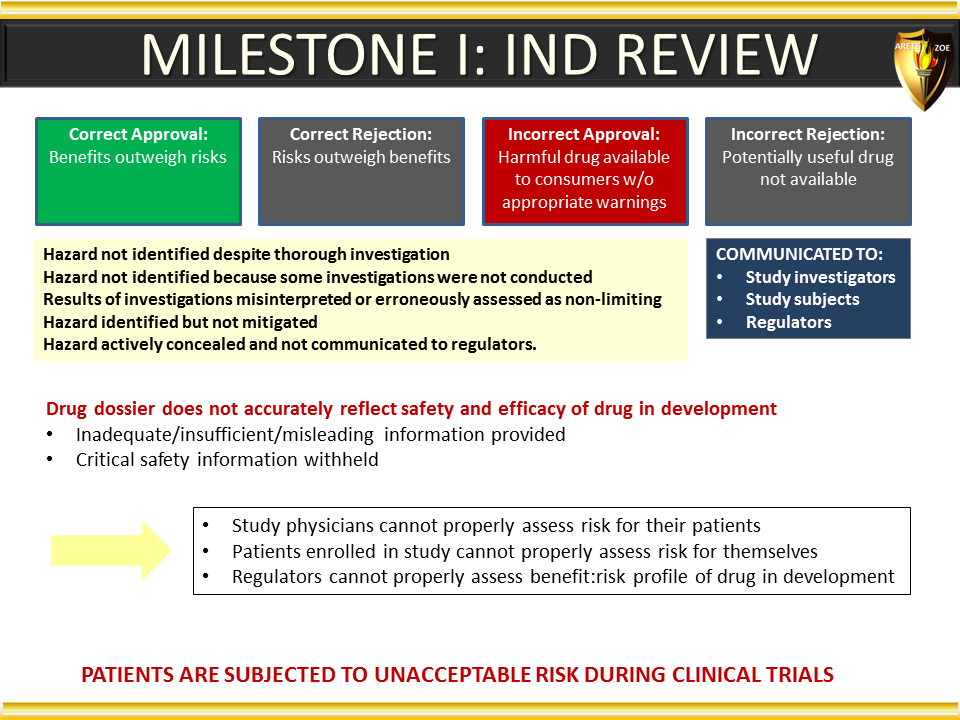
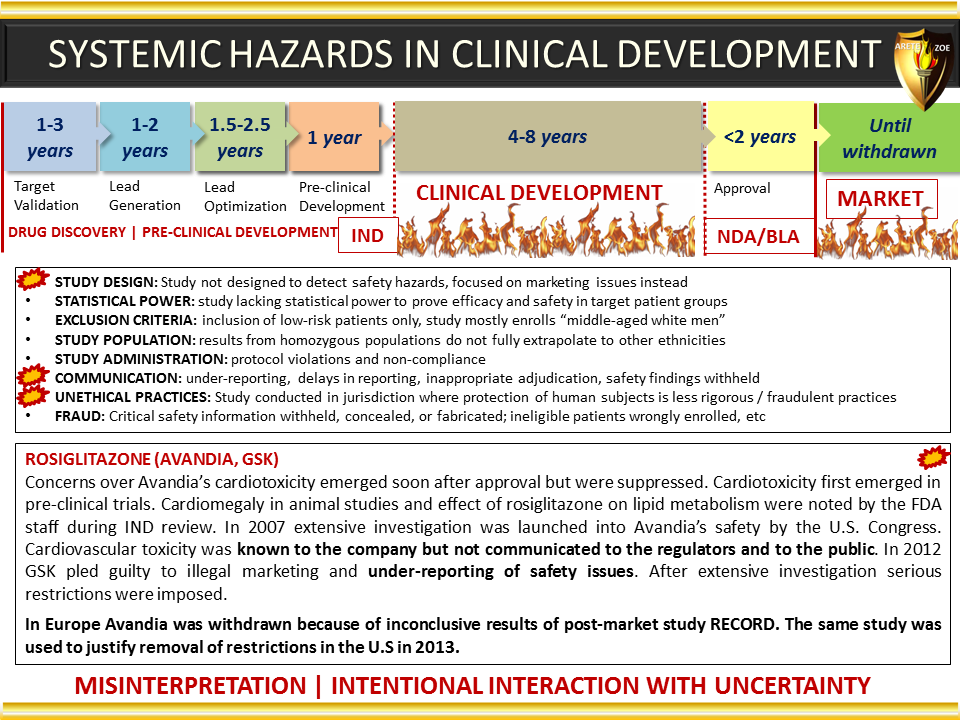
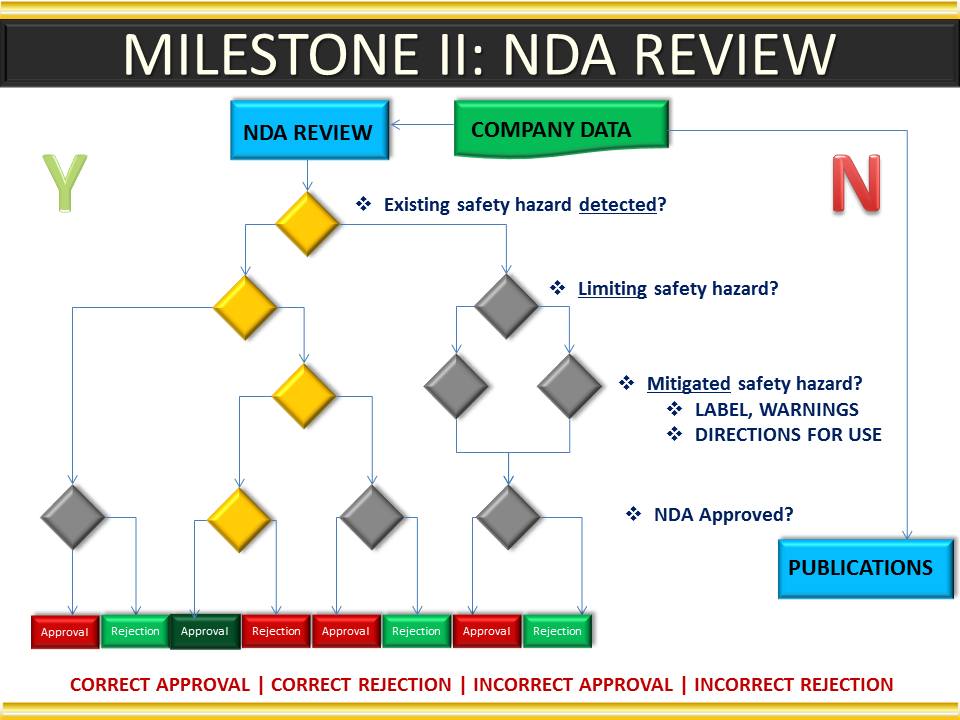
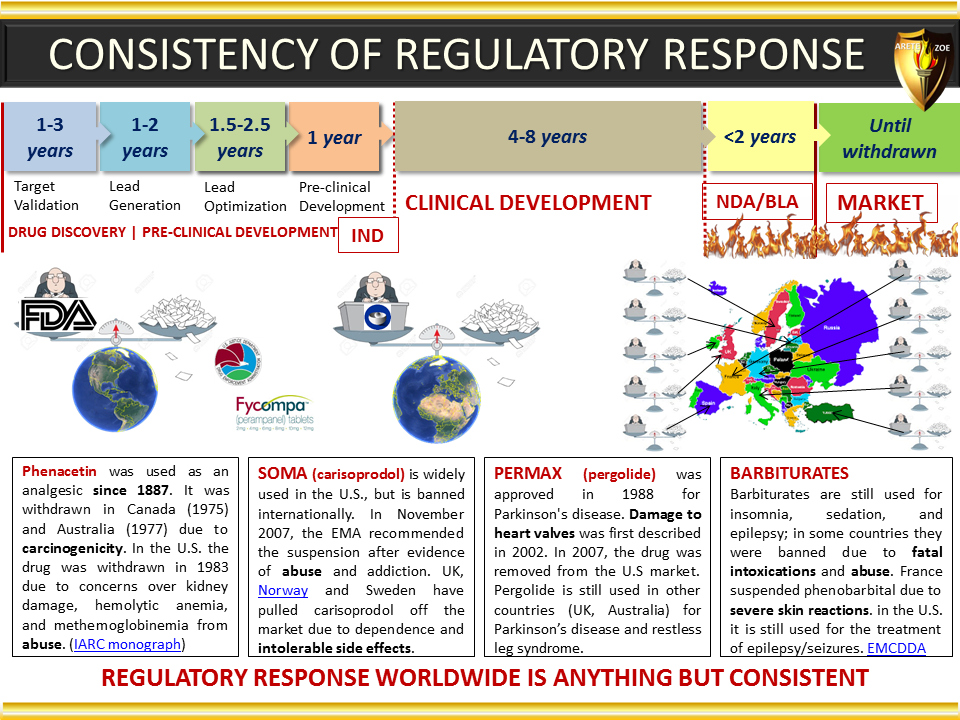
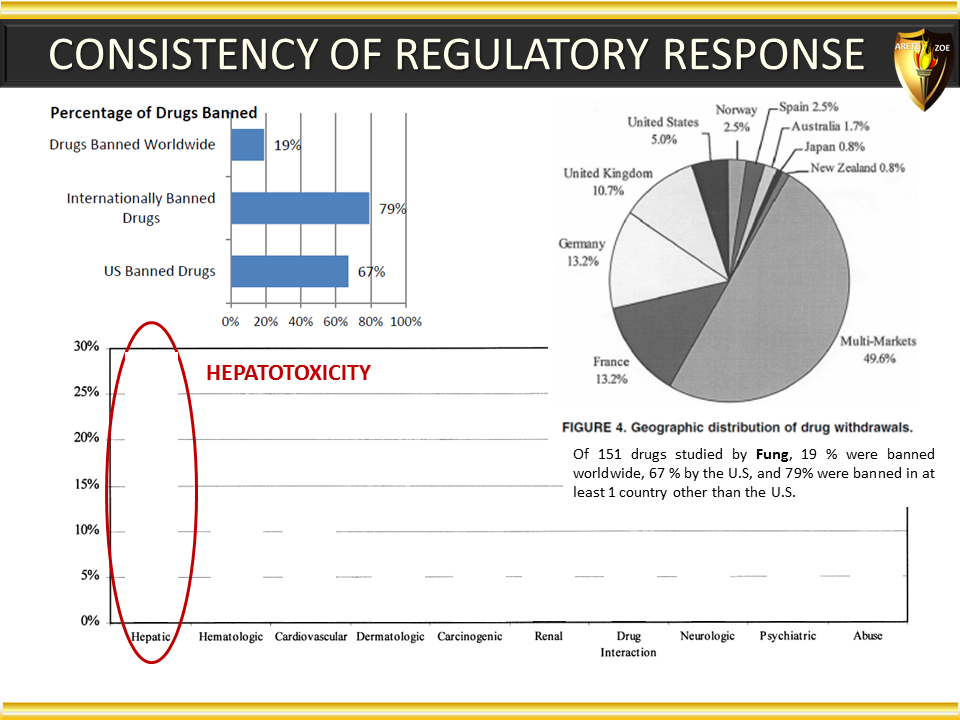
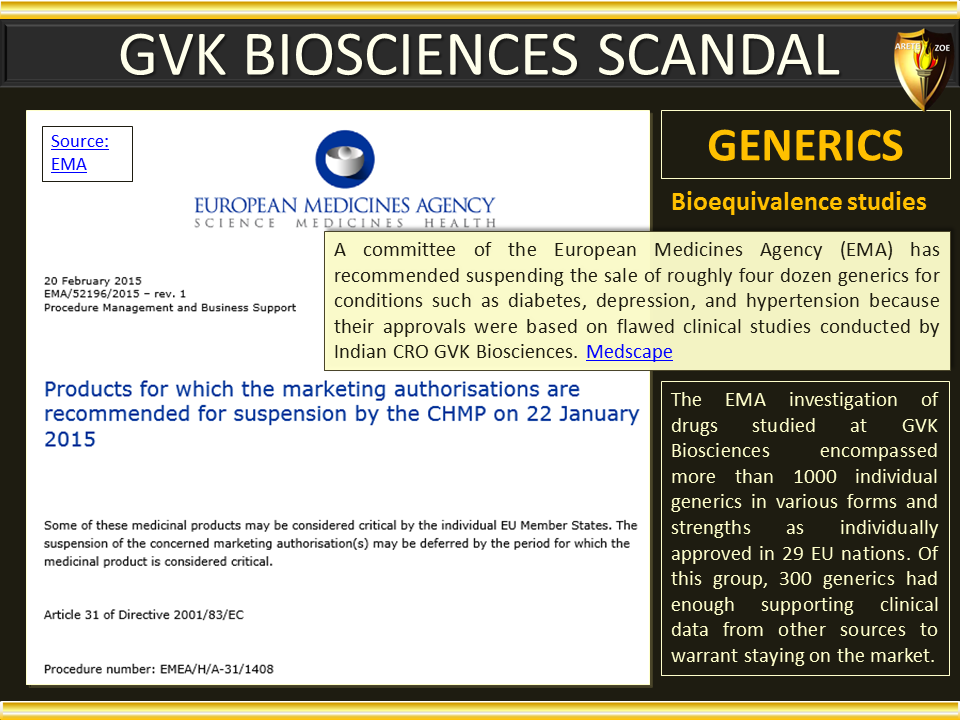
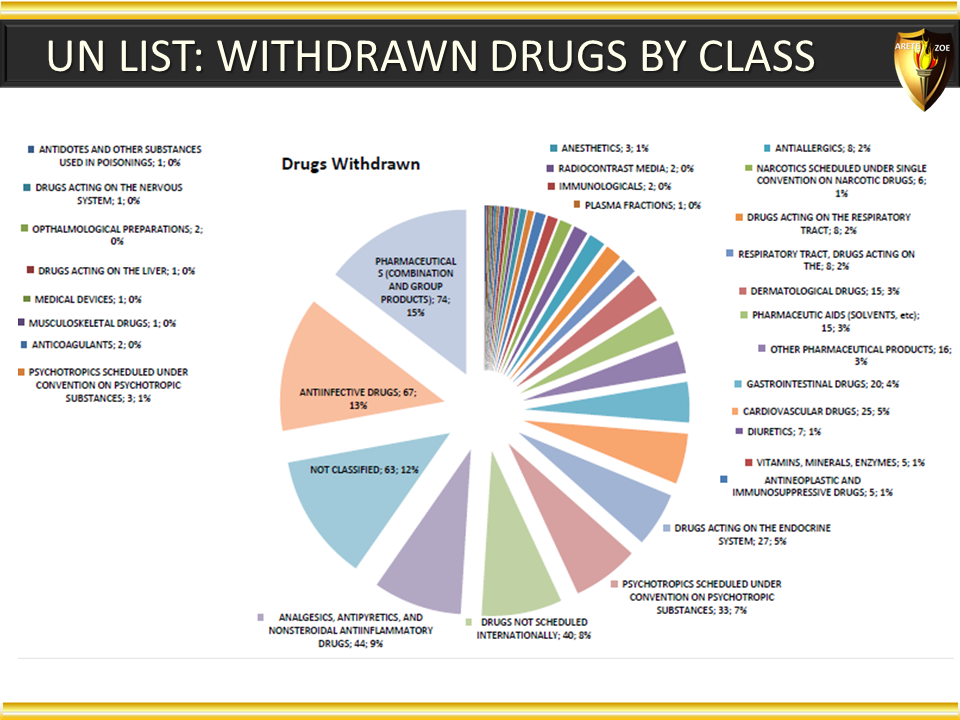
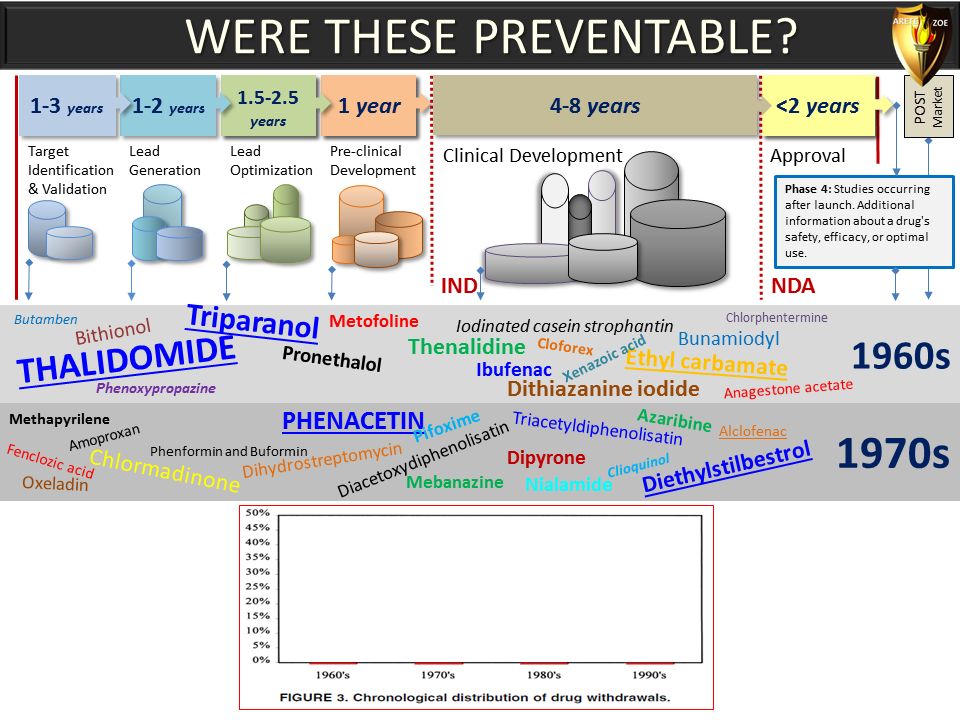
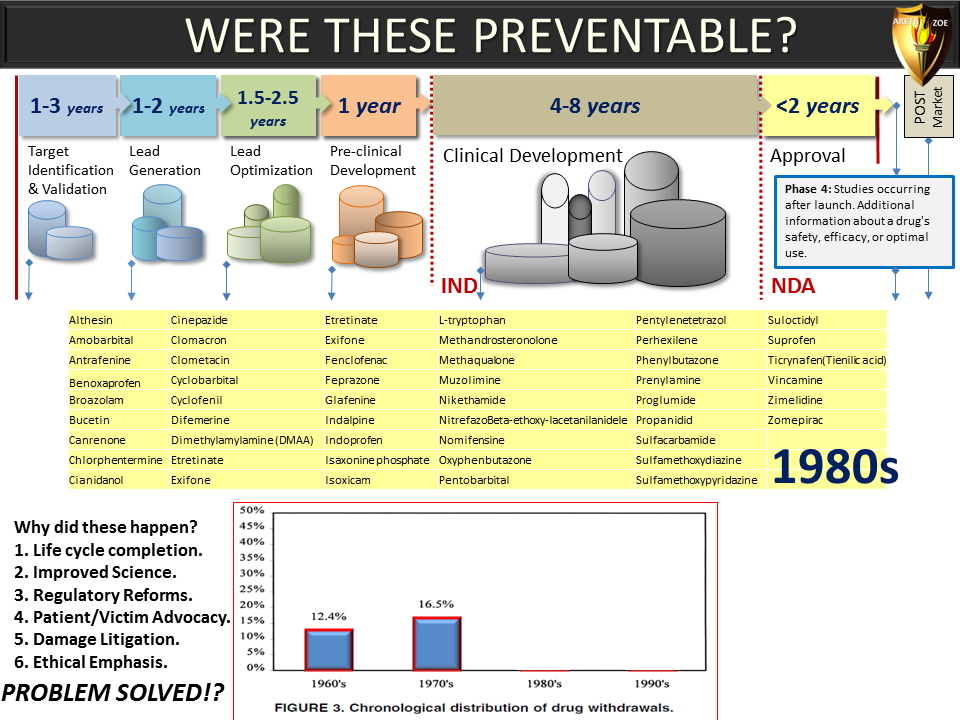
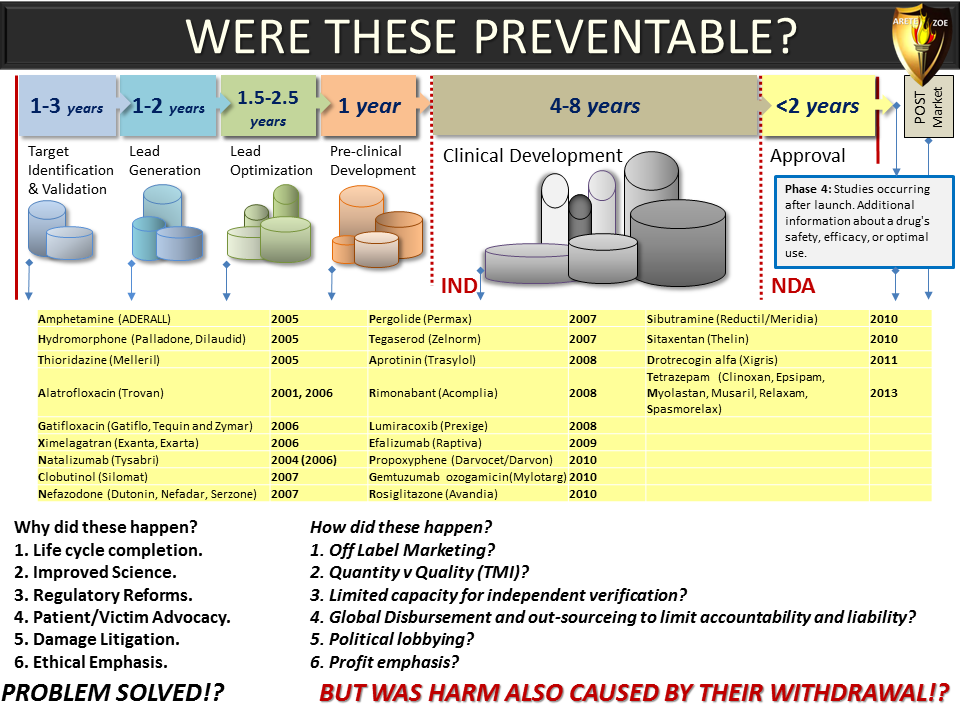
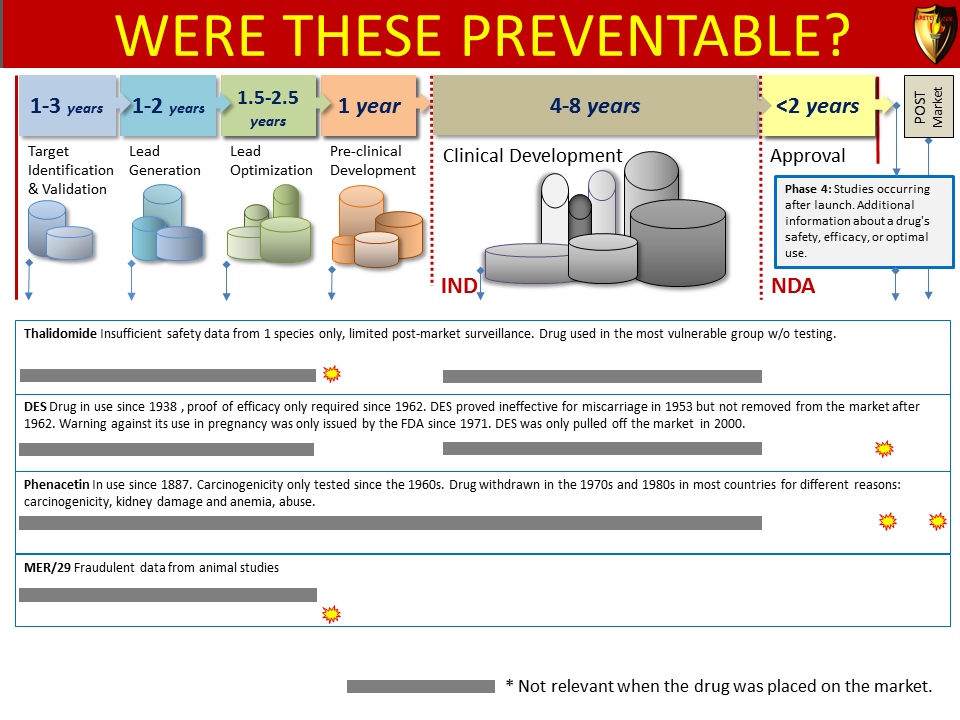
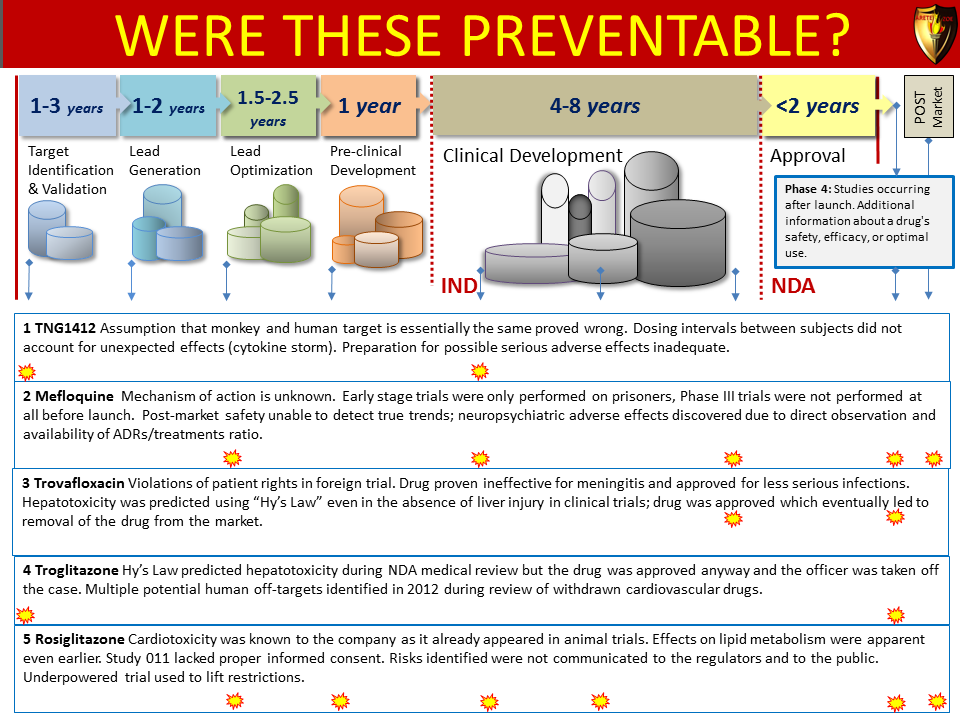
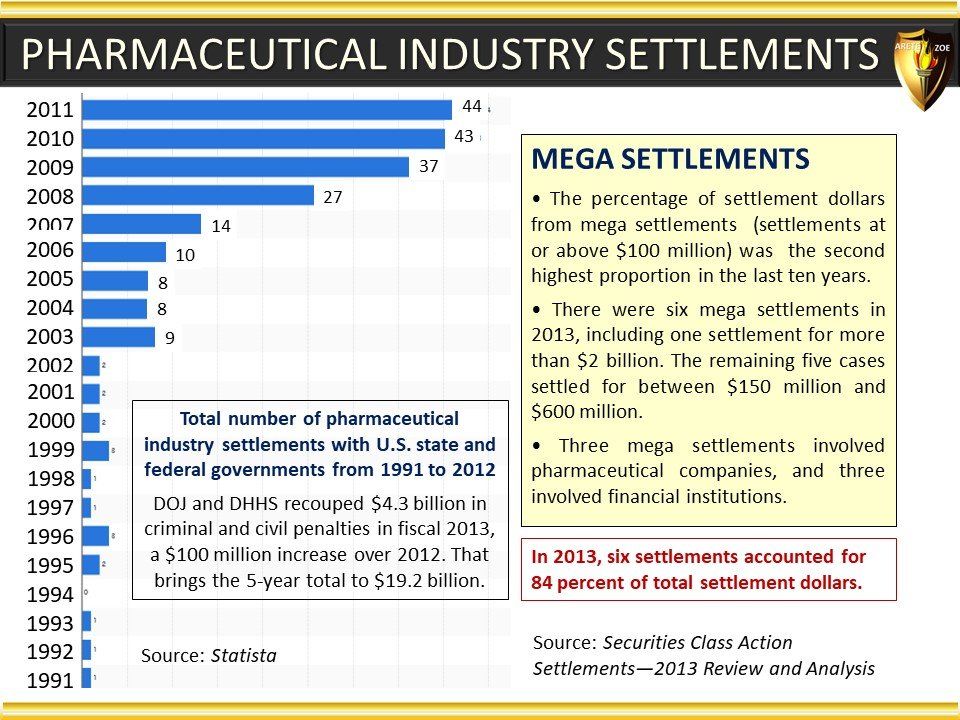
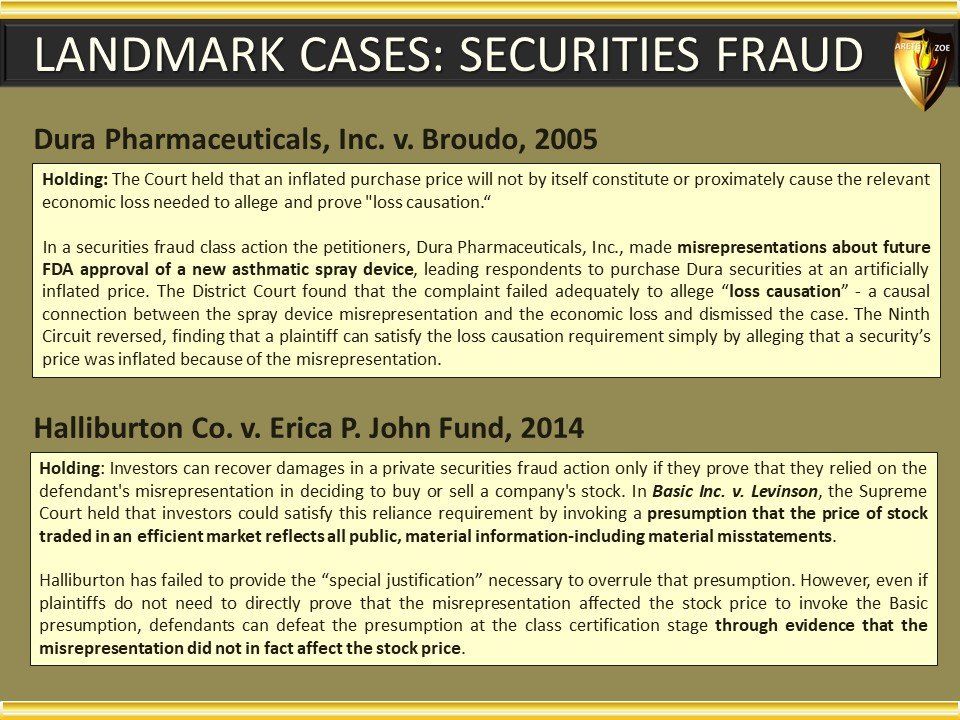
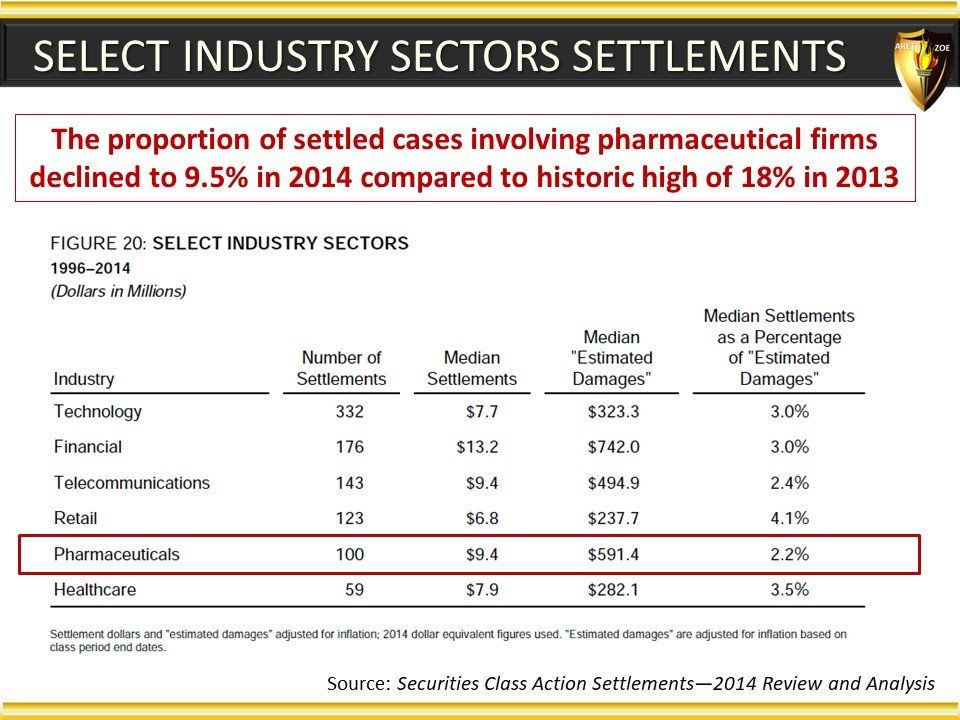
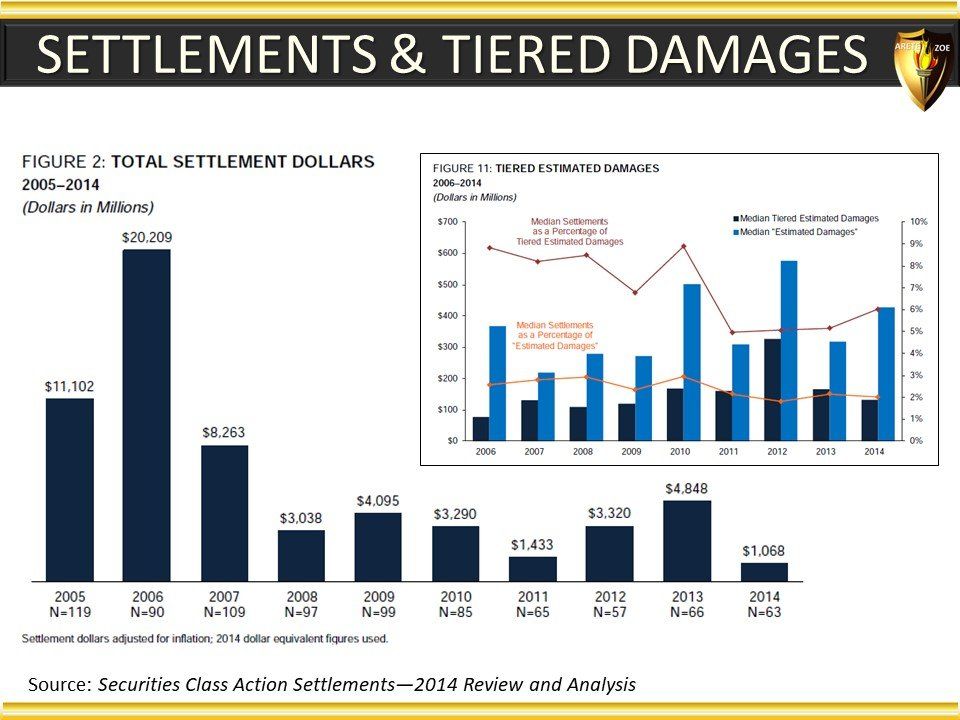
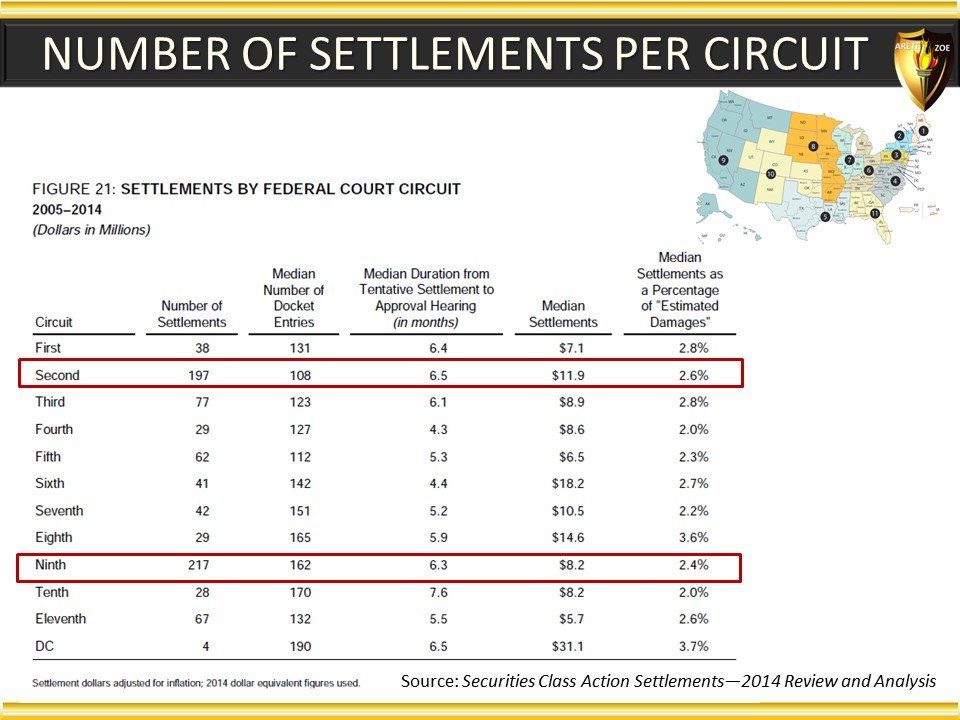
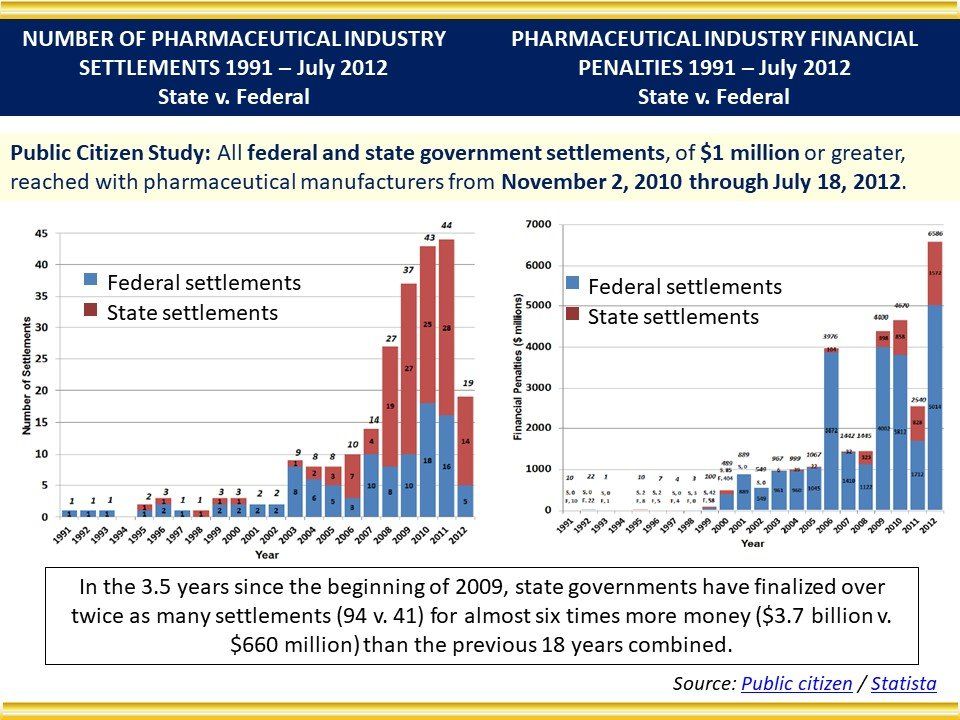
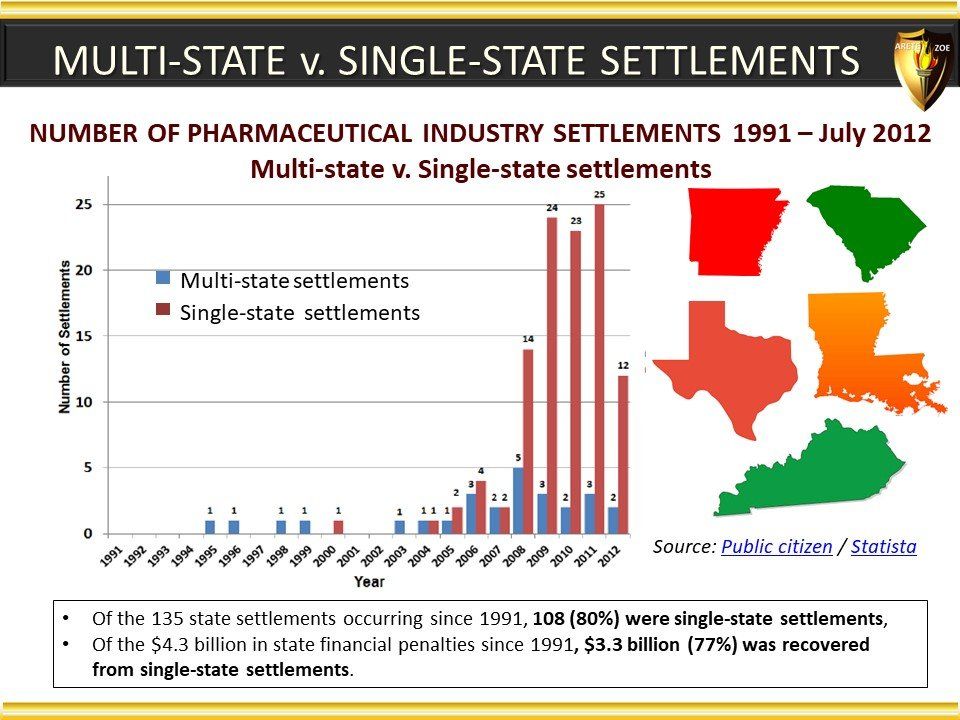
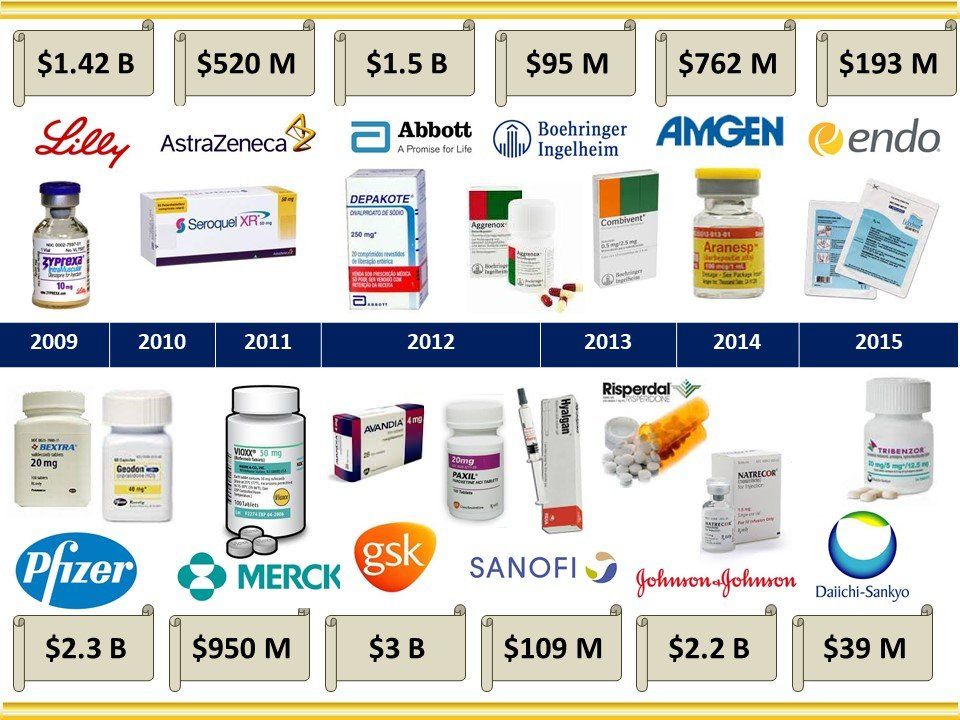
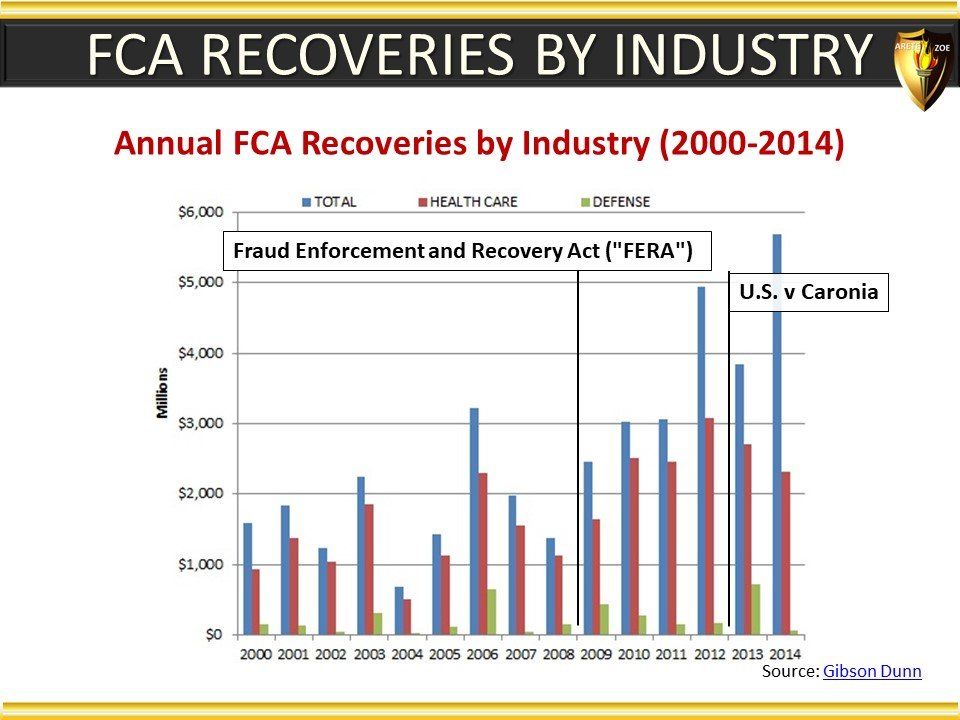
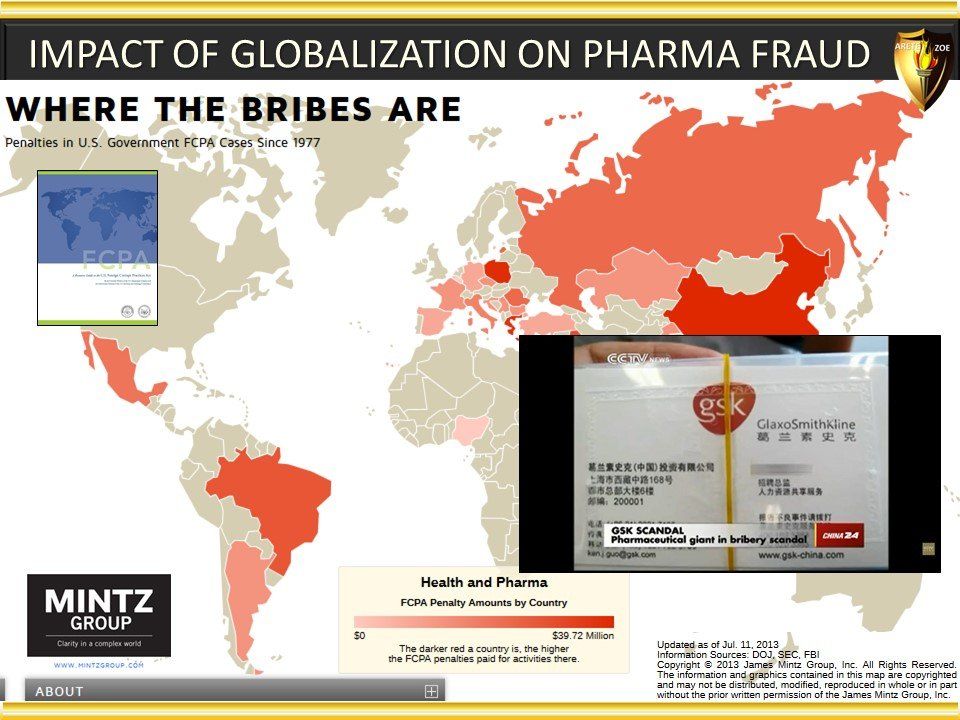
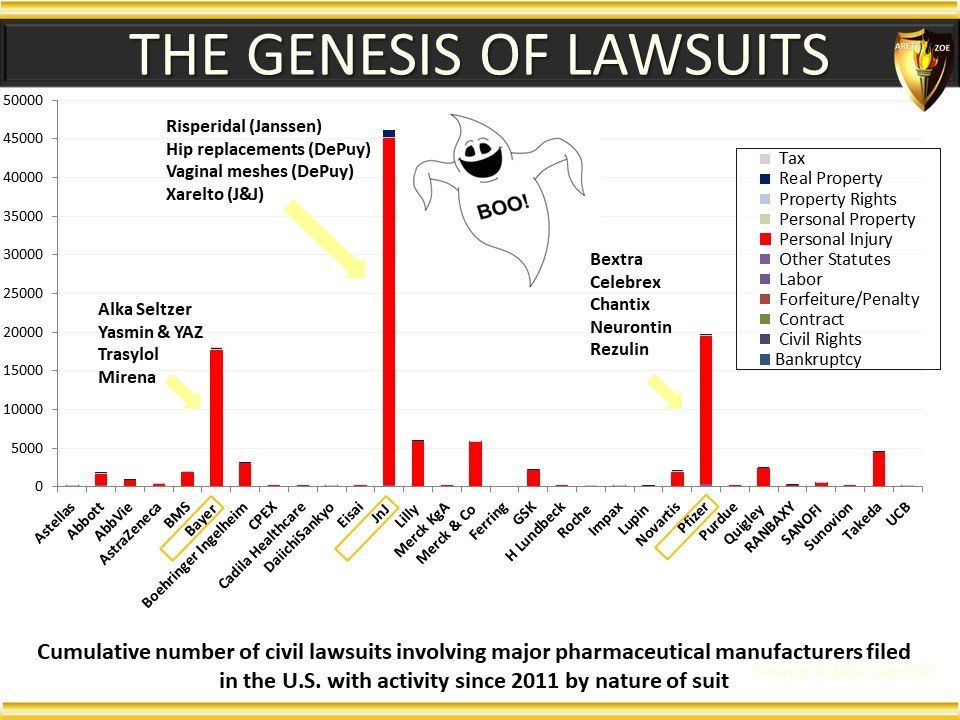
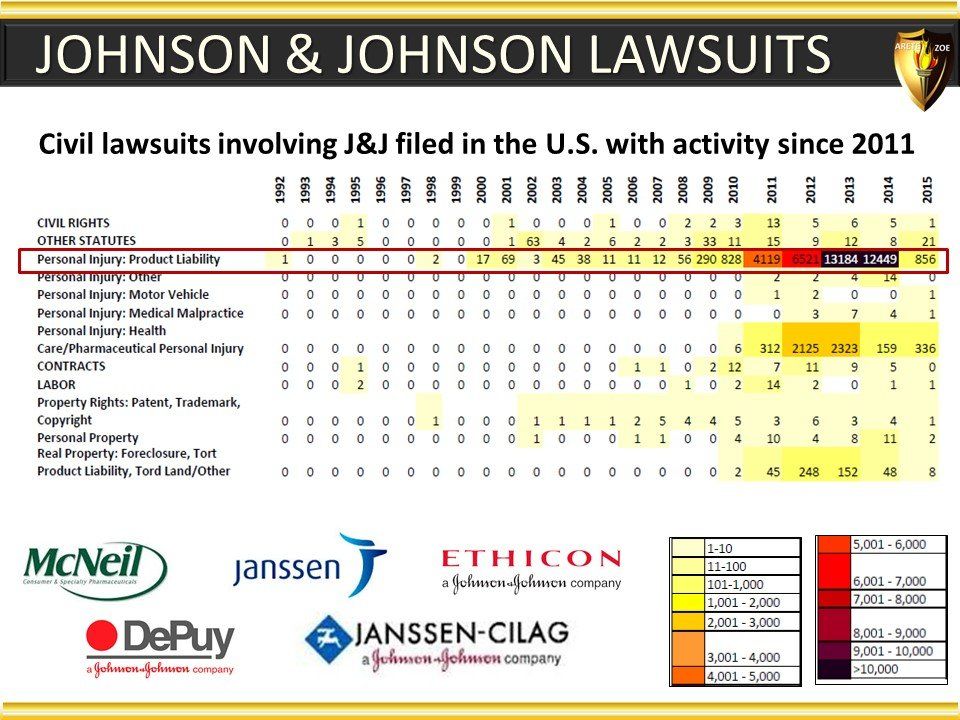
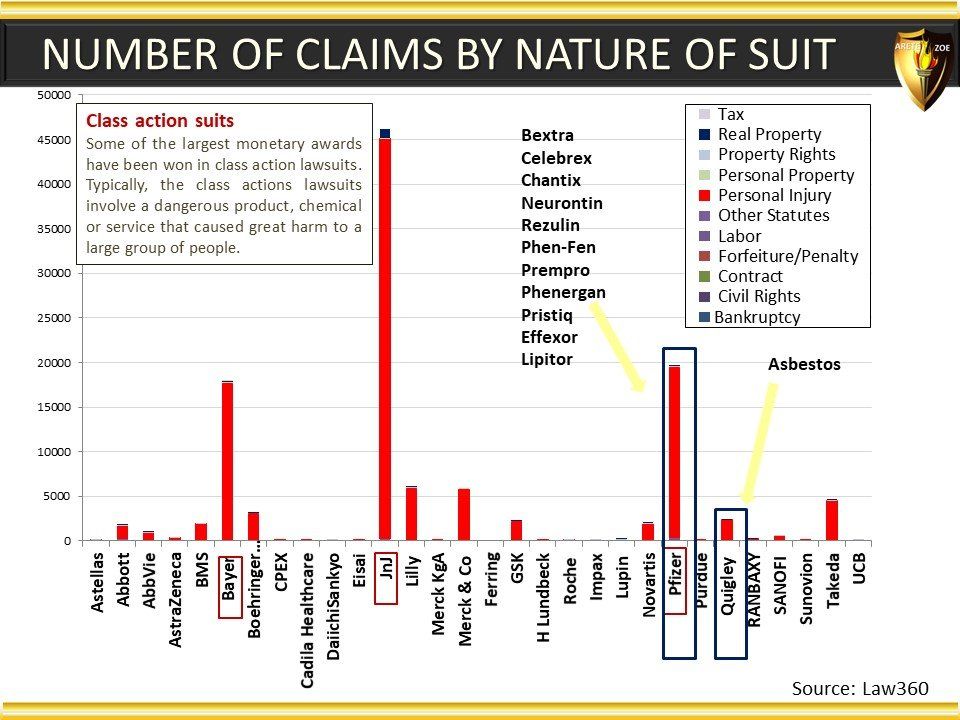
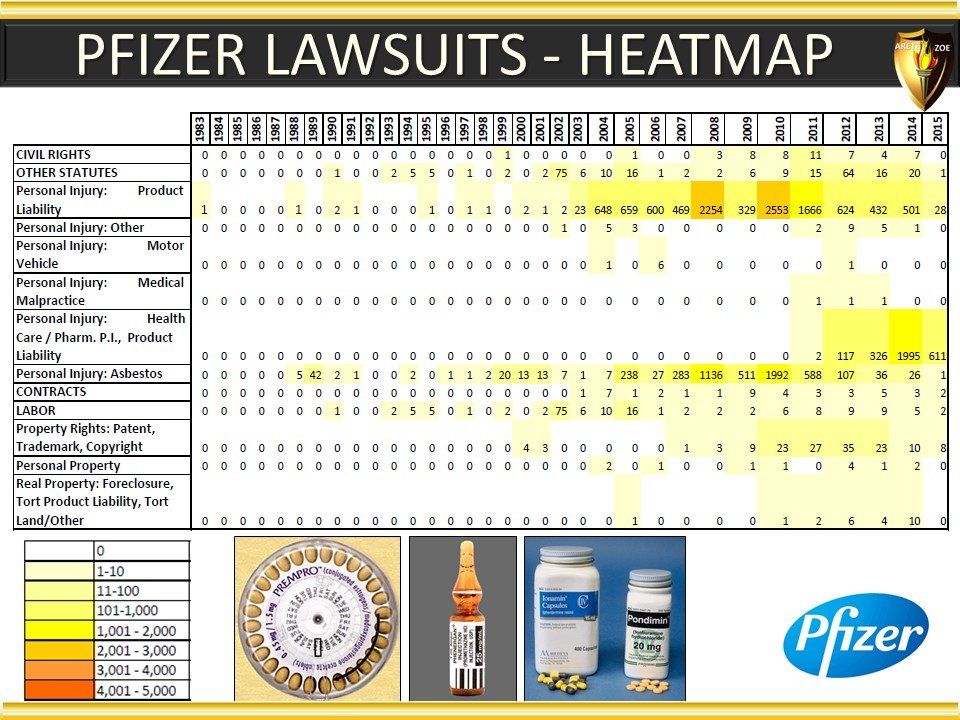
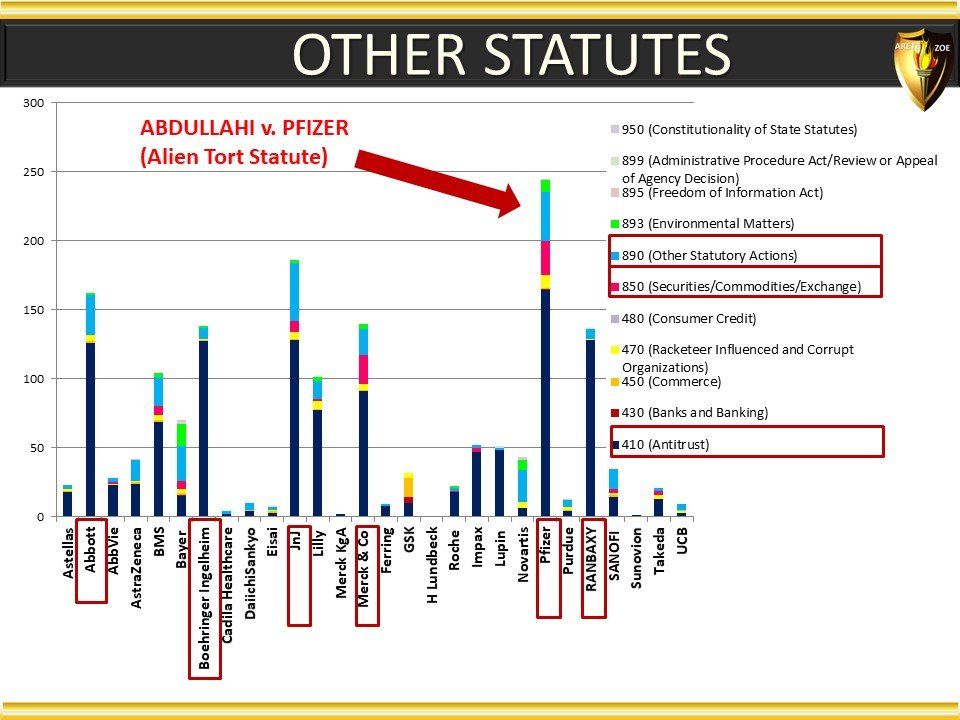
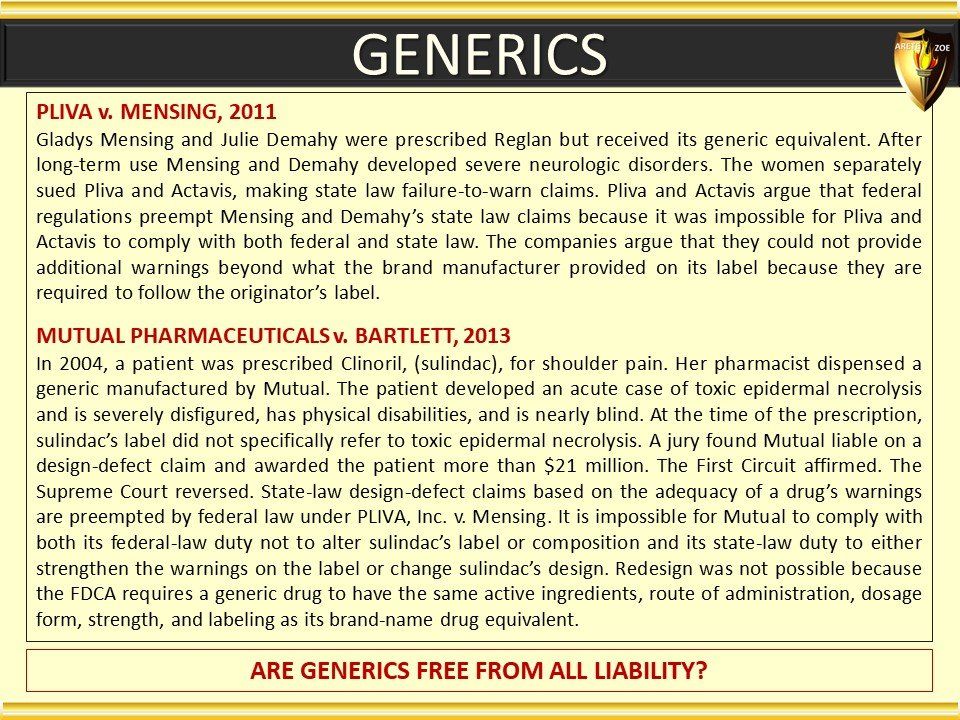
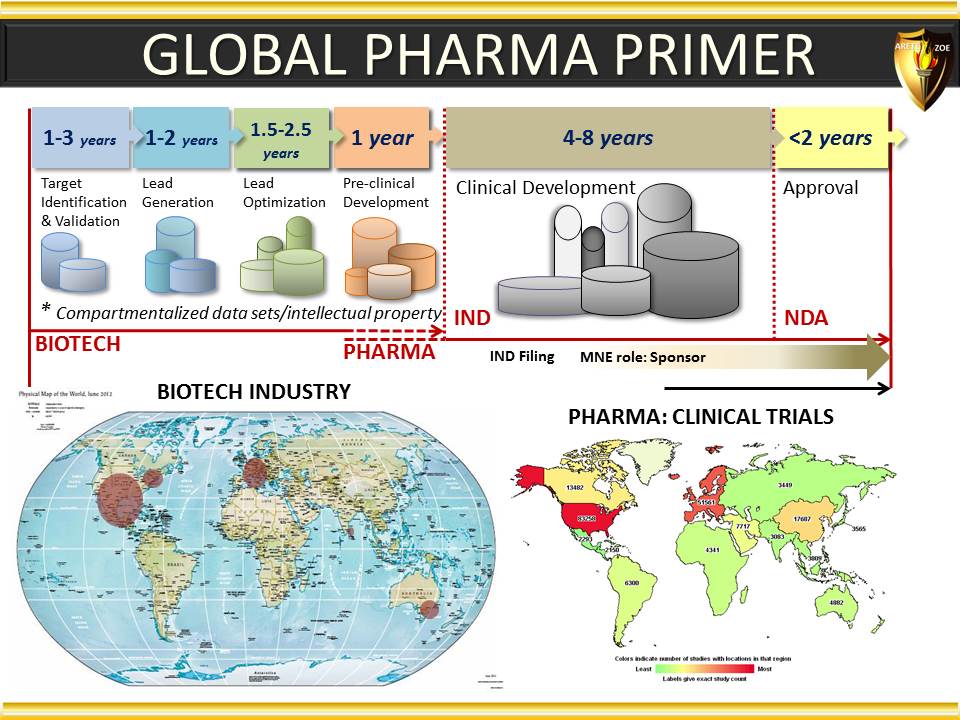
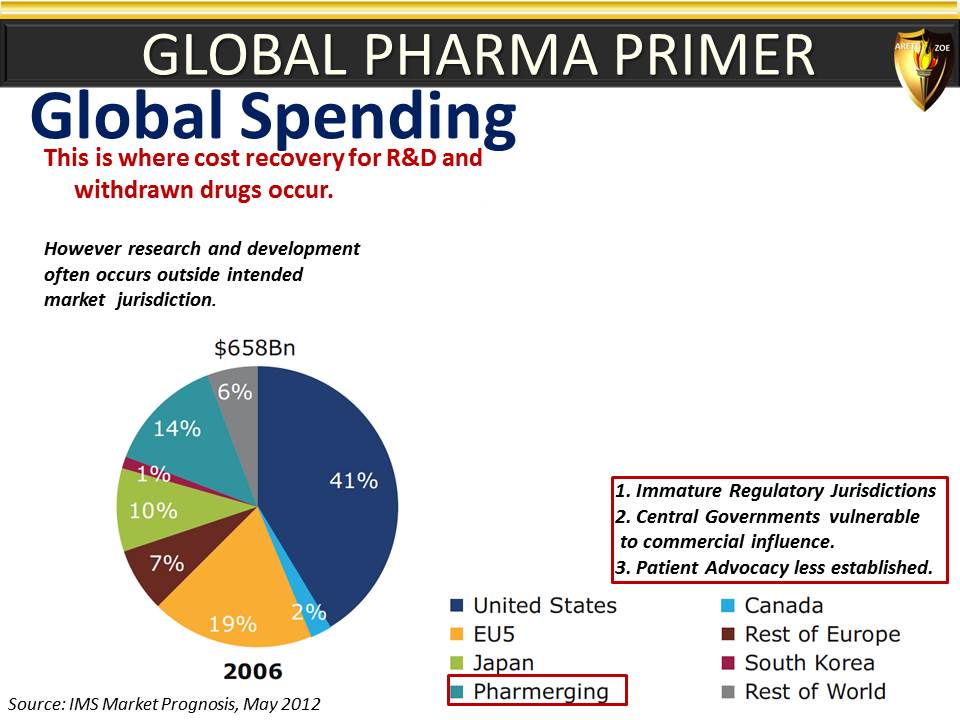
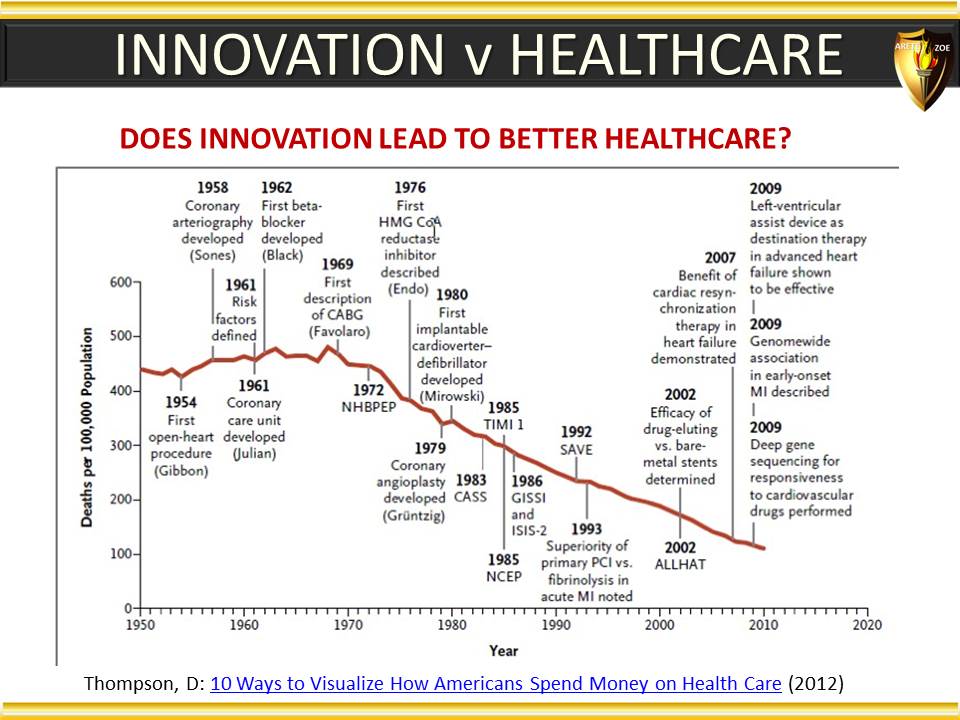
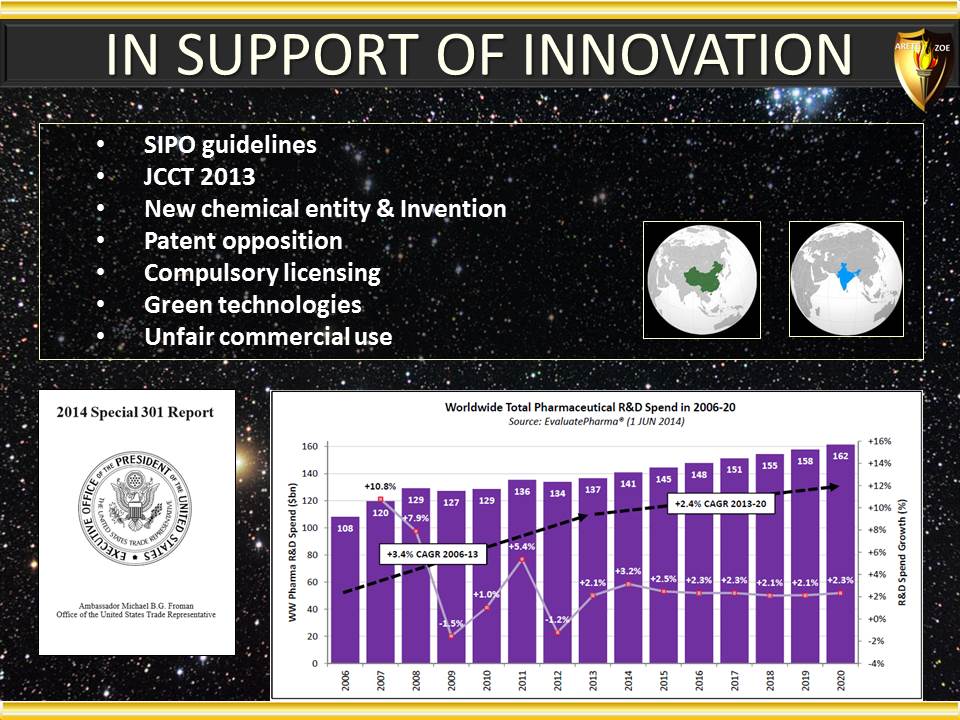
Global Pharma Primer (2015)
Global Pharma Primer: Syllabus and Road Map
Global Pharma Primer: Syllabus and Road Map
Module 1 - Human crisis in pharmaceutical industry and its implications
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Module 2 - Operational environment in pharmaceutical industry and its function
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Module 3 - Systemic vulnerabilities, risk reduction measures, specific examples
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Module 4 - Legal battles in pharmaceutical industry and why they matter
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
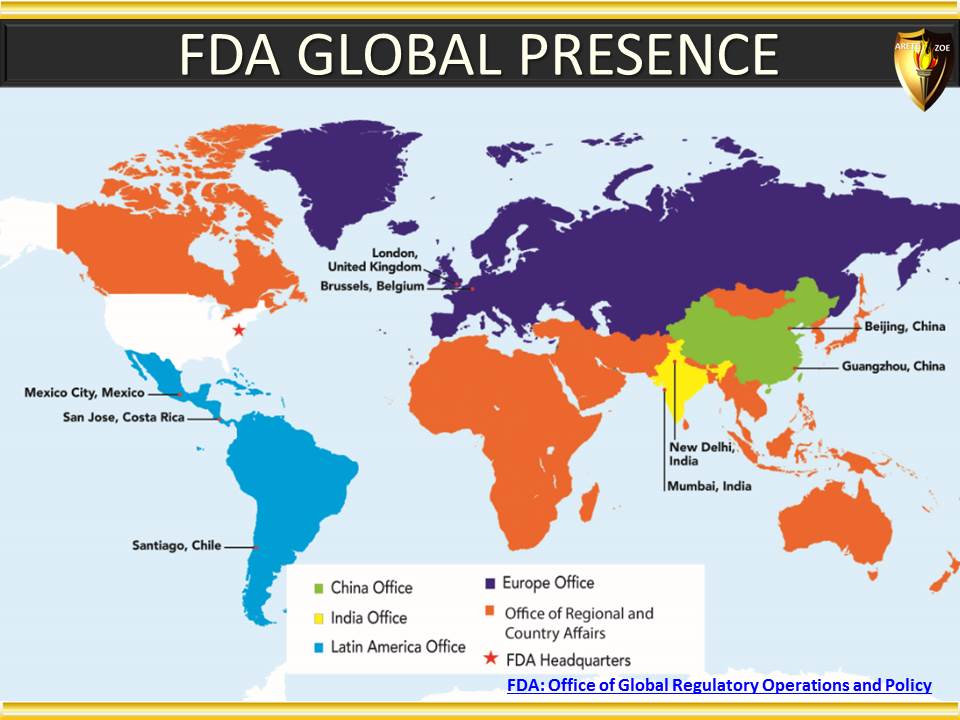
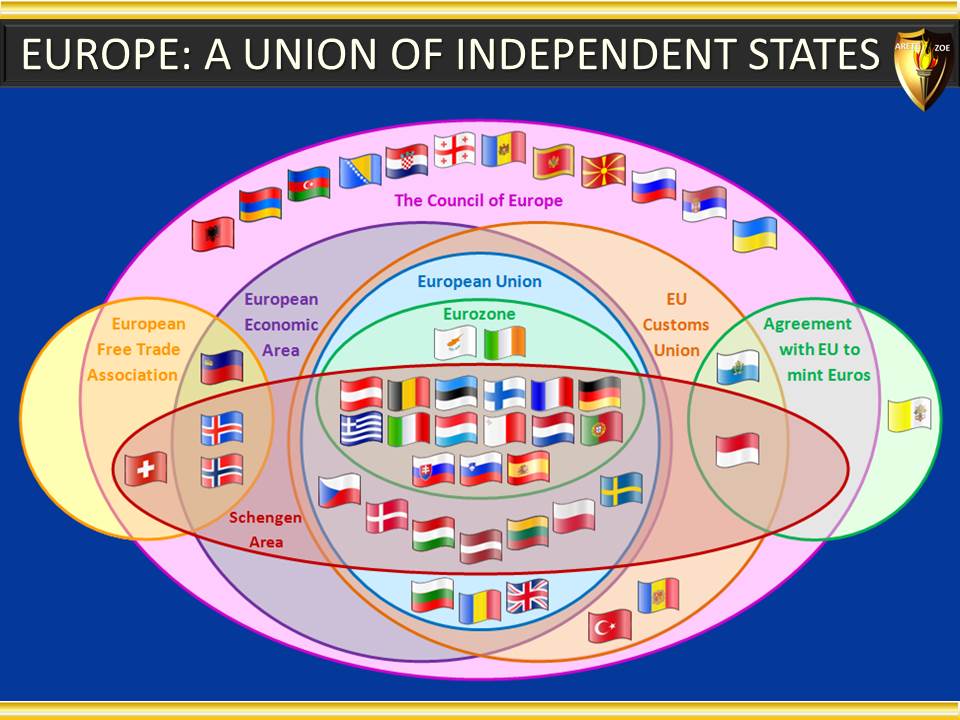
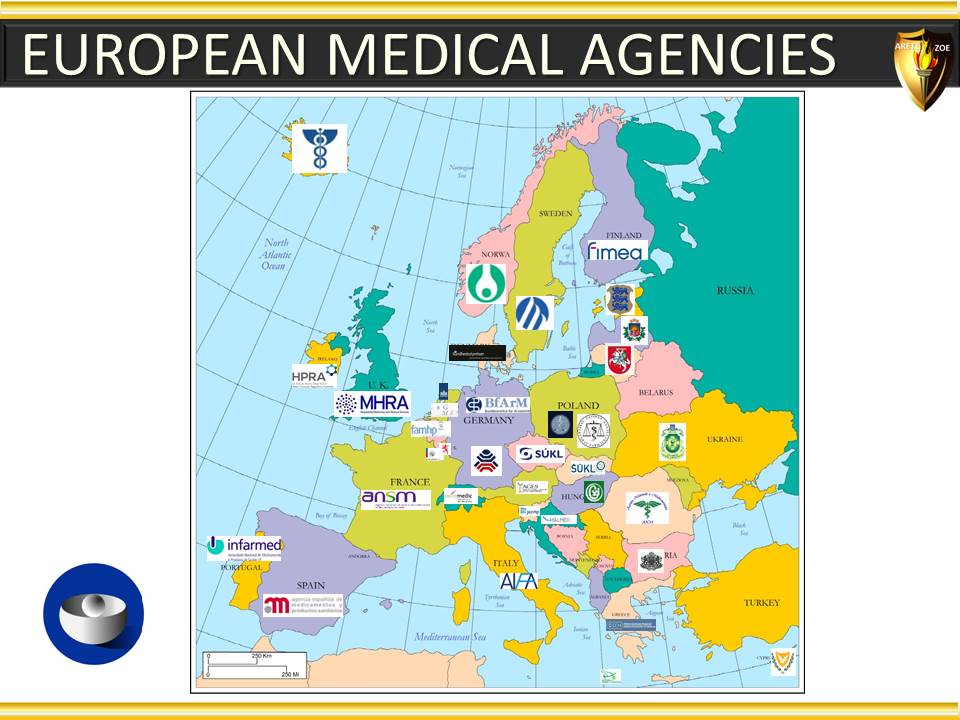
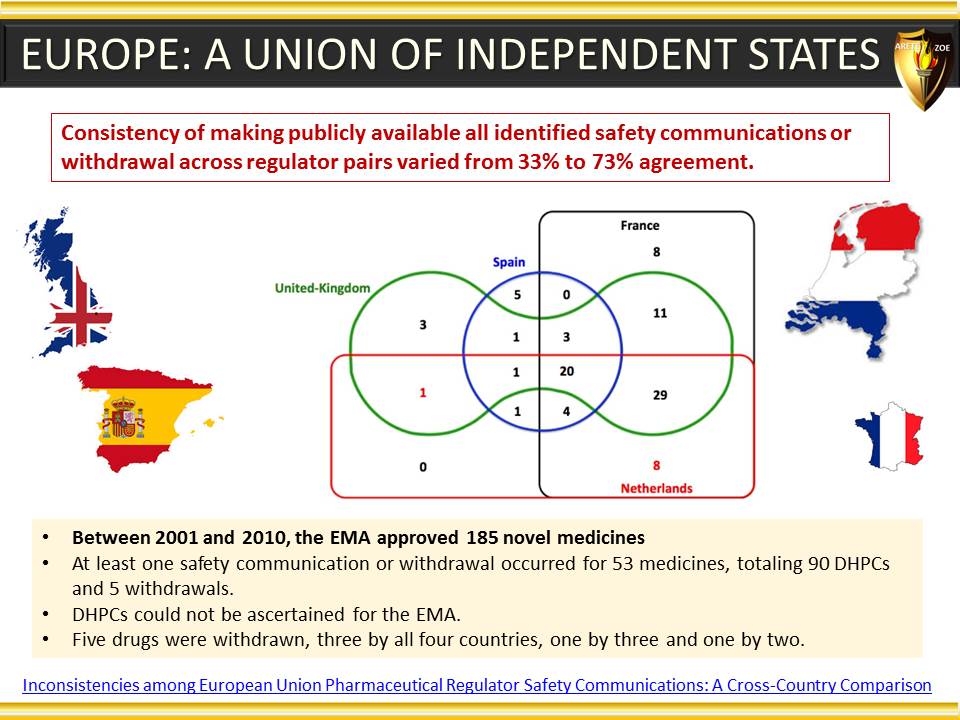
Module 5 - Geographic considerations in pharmaceutical industry, and why they matter
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton